synTQ
About synTQ
synTQ (synchronised Total Quality) is a leading knowledge manager and quality-centric control software suite that empowers manufacturers to achieve smarter, faster, and more consistent production. By unifying real-time data, process control, and advanced analytics, synTQ helps organizations accelerate product development, optimize manufacturing efficiency, and ensure regulatory compliance, all within a single, scalable system trusted by industry leaders worldwide.
Reduce waste, giveaway, and rework
Established more than two decades ago and with more than 200 installations globally, synTQ is now used by ~65% of leading pharmaceutical companies. It is also present in the food, chemical and consumer goods industries.
synTQ’s advanced control strategies have brought significant benefits to the pharmaceutical and related industries, including accelerating product development and enhancing the efficiency of late development and manufacturing.
For one leading biopharmaceutical company, using Process Analytical Technology (PAT) in their process has led to their titer being tripled in a batch process, with further gains anticipated as their continuous improvement learnings are implemented. Another top pharmaceutical company used process analytical technology and synTQ to shorten their 30-day batch manufacturing process to a 90-minute continuous manufacturing one.
- Award-winning knowledge management and quality-centric control software suite
- ISO 9001 ISO 27001 accredited; GxP and 21CFR part 11 compliant
- Agnostic connectivity with new adapter modules added frequently
- Integration with SciY’s digitalization platform
Real-Time Process Control and Data Integration
synTQ enables orchestration and synchronization of disparate equipment and instrumentation, with standard, modular and universal hardware and software integration. With synTQ, you can collect and analyze the real-time data from the various instrumentation utilized in the development and production stages of your manufacturing processes. synTQ adds the layer of 21 CFR part 11 and data integrity compliance to your processes, enabling your instrumentation to run under GLP and GMP.
Advanced control strategies with synTQ can be employed from the laboratory and small-scale manufacture, through to full-scale GMP production, with real-time univariate and multivariate data recording, management, knowledge generation and multivariate statistical process control (MSPC).
Intelligent Process Optimization
Production processes can be controlled with quality predictions using the synTQ platform, which plant operators (and automation systems) can use to develop a mechanism for continuous improvement. The process understanding provided by synTQ can be used to modify process conditions – making the manufacture of high-quality products faster, more reliable and more repeatable.
Vendor-Agnostic Connectivity and Flexibility
synTQ is an instrument vendor agnostic platform that can connect with any device that has a communications interface and a known protocol. synTQ supports a complete process analytical implementation, connecting instrumentation, MVA packages, automation systems or other third-party systems (such as MES, historian, LIMS) from any vendor. The flexibility of synTQ allows its users to select the control equipment that serves them best. The system is also scalable in process, supporting scale-up and scale-out, and also in application, allowing new modules to be added as it grows and evolves.
Free eBook:
‘QbD & PAT for Dummies’ offers a simple and easy to follow guide on what Quality by Design (QbD) and PAT are, the regulatory framework supporting these approaches, as well as how leveraging these concepts can positively transform production processes and quality testing.
Scalable Solutions for Every Stage
synTQ supports lab automation, batch processes, continuous manufacturing, and process-on-demand. This is achieved through process analytical technology (PAT) and the automated, multi-instrument, holistic quality assurance enabled by synTQ.
There is tailored functionality for each stage of the research, development and manufacturing process, via different synTQ editions. With options that range from solutions for small systems with single unit operations (for early investigative projects, for example), to plant-wide distributed systems running multiple unit operations all over the world.
The ability to easily expand your version or edition of synTQ gives you the confidence to choose a system that suits your needs today and will cover your needs tomorrow.
Your Challenges – Solved
- Real-time and offline analysis
for quality assurance, process feedback and control - Validation and compliance burden
Quality by Design manufacturing processes require a vendor-agnostic and GMP compliant knowledge management system that interfaces with the analytical equipment that you need. - Method lifecycle management
Continuous improvement of methods ensures optimal production conditions and capacity. This can be enhanced with simulation or digital twin functions. - Online, offline, at-line or in-line?
Different and multiple measurement approache can be correlated and contextualized in the same data management system.
- Limited instrument connectivity
synTQ has agnostic connectivity to a huge array of instruments, MVA packages and control systems. - Unconnected islands of digitalization
A single digitalization platform that connects with a myriad of technologies enables clear, simple and effective methodologies. - Scale up and scale out
Implementing control feedback and continuous improvement from synTQ to the manufacturing control system using the same platform allows you to easily transition from the pilot plant to enterprise-wide GMP manufacturing, using your own validated methodologies.
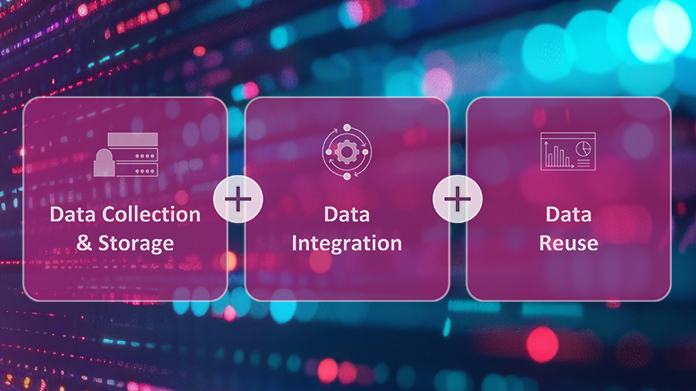
synTQ – Complete Process Knowledge Management
Visualize historic and real-time data with configurable charting tools and dashboards
Real-time and offline analysis for quality assurance, process feedback and control
Design, control and store methods within the GxP compliant, secure knowledge management system
synTQ orchestrates different instruments and sensors. No-code, just drag & drop to build your workflows and enable feedback control
synTQ dashboards allow for accurate process control. Bring the process back to its sweet spot!
Curious about our products or services? Let us provide you with the answers you need. Contact us today and discover how we can cater to your needs effectively.